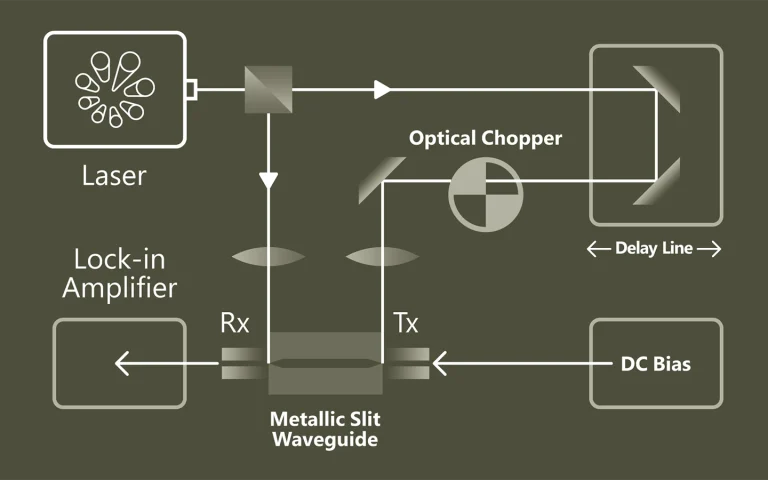
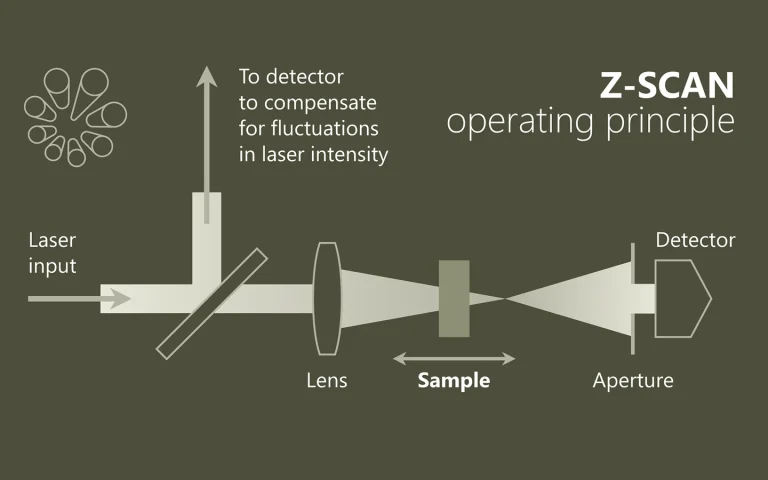
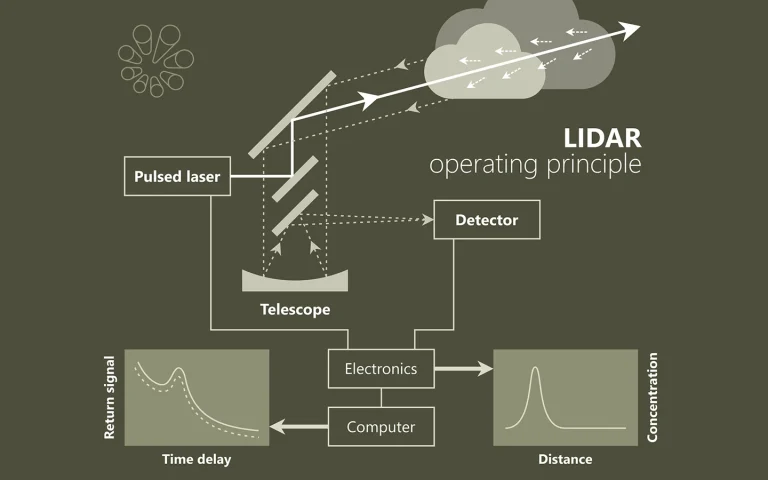
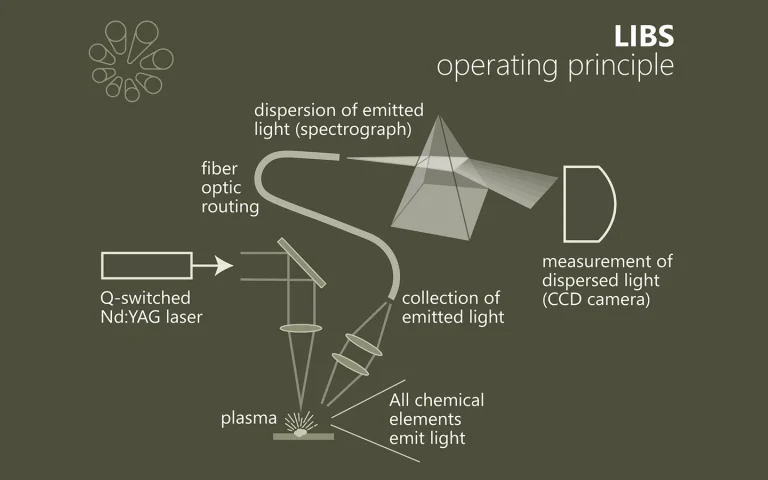
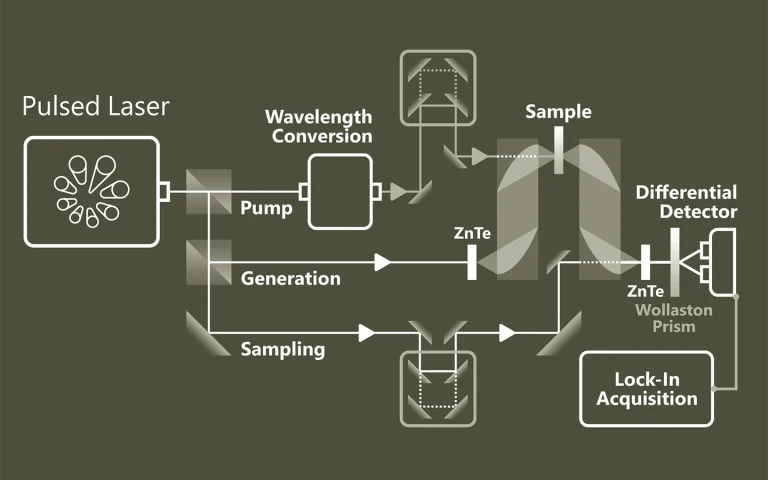
Laser spectroscopy
For a long time laser spectroscopy has been source of inspiration for EKSPLA scientific laser engineers. Explore some typical applications where our picosecond and nanosecond lasers has been employed.
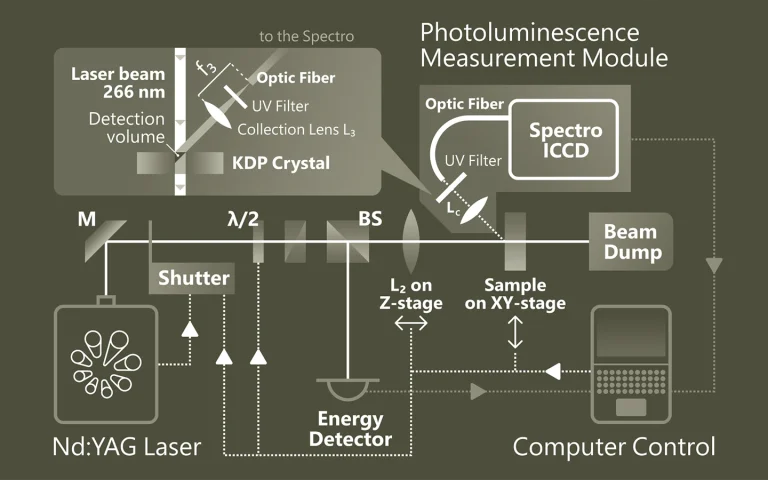
Solid-phase photoluminescence spectroscopy
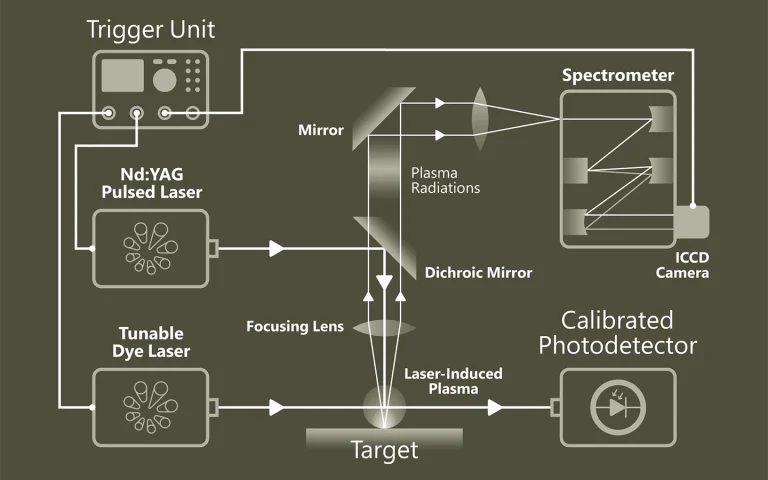
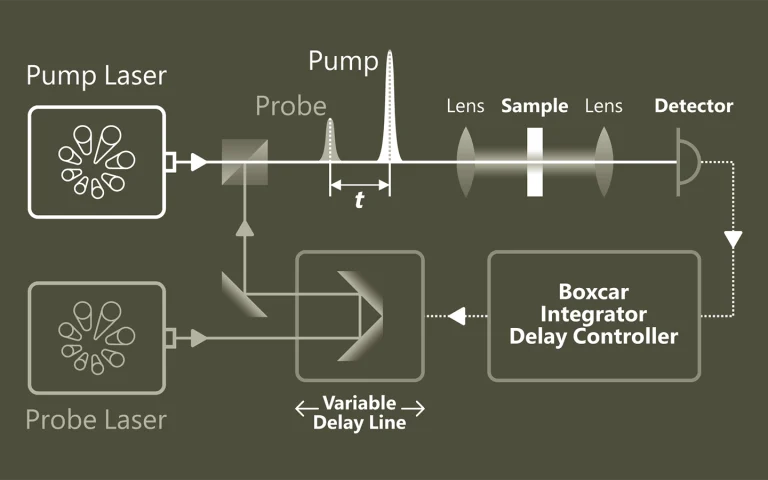
Time-resolved Photoluminescence Spectroscopy (TRPL) is a contactless method to characterize recombination and transport in solid materials. Measuring TRPL requires exciting luminescence from a sample with a pulsed light source and then measuring the subsequent decay in photoluminescence as a function of time. Most experiments excite the sample with a pulsed laser source and detect the PL with a photodiode, streak camera, or photomultiplier tube set up for upconversion or single-photon counting. The system response time, wavelength range and sensitivity vary widely for each configuration.
It is possible to apply the general methodology of time-resolved photoluminescence for lifetime imaging of the charge carrier dynamics. Nonradiative surface recombination at the boundaries of a semiconductor device can be a major factor limiting the efficiency in light-emitting and laser diodes (LEDs and LDs), photovoltaic cells, and photodetectors. Therefore, the effective lifetime is a crucial parameter to obtain solar cells with a high conversion ratio.
Photoluminescence microscopy also is a powerful optical method for the study of crystal defects in semiconductors and organometallic complexes, with important applications in the manufacturing process of nanostructures, optoelectronic devices and solar cell systems.
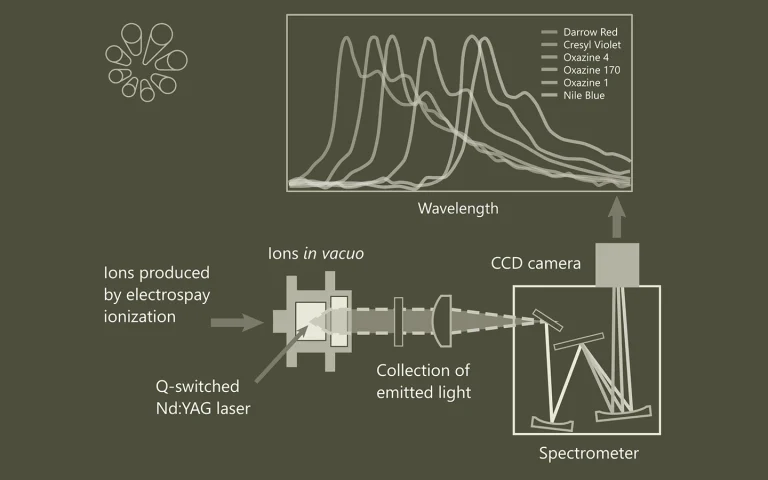
Luminescence spectroscopy of oxazine dye cations isolated in vacuo
Here we report gas-phase action and luminescence spectra of cationic dyes derived from oxazine: cresyl violet (CV+), oxazine 170 (Ox-170+), nile blue (NB+), darrow red (DR+), oxazine 1 (Ox-1+), oxazine 4 (Ox-4+), and brilliant cresyl blue (BCB+). The first four have a benzofused structure, which results in asymmetric charge distributions along the long axis. The positive charge is also asymmetrically distributed in BCB+ while Ox-1+ and Ox-4+ are symmetric. As the ions are isolated in vacuo, there are no interactions with solvent molecules or counter ions, and the effect of chemical modifications is therefore more easily revealed than from solution-phase experiments. The transition energy decreases in the order: DR+ > CV+ > Ox-4+ > Ox-170+ > BCB+ > Ox-1+ > NB+, and the fluorescence from BCB+ is less than from the others. We discuss the results based on electron delocalisation, degree of charge-transfer character, rigidity of the chromophore structure, and substituents.
How nature covers its bases
The response of DNA and RNA bases to ultraviolet (UV) radiation has been receiving increasing attention for a number of important reasons: (i) the selection of the building blocks of life on an early earth may have been mediated by UV photochemistry, (ii) radiative damage of DNA depends critically on its photochemical properties, and (iii) the processes involved are quite general and play a role in more biomolecules as well as in other compounds. A growing number of groups worldwide have been studying the photochemistry of nucleobases and their derivatives. Here we focus on gas phase studies, which (i) reveal intrinsic properties distinct from effects from the molecular environment, (ii) allow for the most detailed comparison with the highest levels of computational theory, and (iii) provide isomeric selectivity. From the work so far a picture is emerging of rapid decay pathways following UV excitation. The main understanding, which is now well established, is that canonical nucleobases, when absorbing UV radiation, tend to eliminate the resulting electronic excitation by internal conversion (IC) to the electronic ground state in picoseconds or less. The availability of this rapid “safe” de-excitation pathway turns out to depend exquisitely on molecular structure. The canonical DNA and RNA bases are generally short-lived in the excited state, and thus UV protected. Many closely related compounds are longer lived, and thus more prone to other, potentially harmful, photochemical processes. It is this structure dependence that suggests a mechanism for the chemical selection of the building blocks of life on an early earth. However, the picture is far from complete and many new questions now arise.
Near infrared emission properties of Er doped cubic sesquioxides in the second/third biological windows
In the recent years, there is an extensive effort concentrated towards the development of nanoparticles with near-infrared emission within the so called second or third biological windows induced by excitation outside 800 – 1000 nm range corresponding to the traditional Nd (800 nm) and Yb (980 nm) sensitizers. Here, we present a first report on the near-infrared (900 – 1700 nm) emission of significant member of cubic sesquioxides, Er-Lu2O3 nanoparticles, measured under both near-infrared up-conversion and low energy X-ray excitations. The nanoparticle compositions are optimized by varying Er concentration and Li addition. It is found that, under ca. 1500 nm up-conversion excitation, the emission is almost monochromatic (>93%) and centered at 980 nm while over 80% of the X-ray induced emission is concentrated around 1500 nm. The mechanisms responsible for the up-conversion emission of Er – Lu2O3 are identified by help of the up-conversion emission and excitation spectra as well as emission decays considering multiple excitation/emission transitions across visible to near-infrared ranges. Comparison between the emission properties of Er-Lu2O3 and Er-Y2O3 induced by optical and X-ray excitation is also presented. Our results suggest that the further optimized Er-doped cubic sesquioxides represent promising candidates for bioimaging and photovoltaic applications.
Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors
Red-emitting Mn4+-doped fluorides are a promising class of materials to improve the color rendering and luminous efficacy of white light-emitting diodes (w-LEDs). For w-LEDs, the luminescence quenching temperature is very important, but surprisingly no systematic research has been conducted to understand the mechanism for thermal quenching in Mn4+-doped fluorides. Furthermore, concentration quenching of the Mn4+ luminescence can be an issue but detailed investigations are lacking. In this work, we study thermal quenching and concentration quenching in Mn4+-doped fluorides by measuring luminescence spectra and decay curves of K2TiF6:Mn4+ between 4 and 600 K and for Mn4+ concentrations from 0.01% to 15.7%. Temperature-dependent measurements on K2TiF6:Mn4+ and other Mn4+-doped phosphors show that quenching occurs through thermally activated crossover between the 4T2 excited state and 4A2 ground state. The quenching temperature can be optimized by designing host lattices in which Mn4+ has a high 4T2 state energy. Concentration-dependent studies reveal that concentration quenching effects are limited in K2TiF6:Mn4+ up to 5% Mn4+. This is important, as high Mn4+ concentrations are required for sufficient absorption of blue LED light in the parity-forbidden Mn4+ d–d transitions. At even higher Mn4+ concentrations (>10%), the quantum efficiency decreases, mostly due to direct energy transfer to quenching sites (defects and impurity ions). Optimization of the synthesis to reduce quenchers is crucial for developing more efficient highly absorbing Mn4+ phosphors. The present systematic study provides detailed insights into temperature and concentration quenching of Mn4+ emission and can be used to realize superior narrow-band red Mn4+ phosphors for w-LEDs.
Ultra-sensitive mid-infrared emission spectrometer with sub-ns temporal resolution
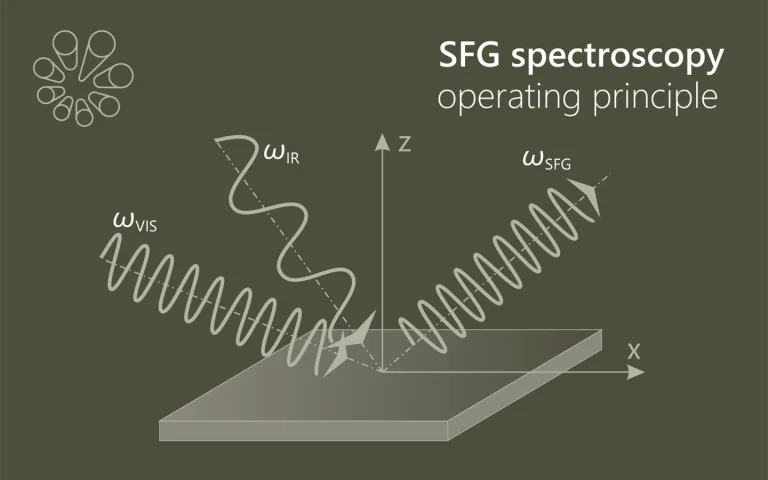
We evaluate the performance of a mid-infrared emission spectrometer operating at wavelengths between 1.5 and 6 μm based on an amorphous tungsten silicide (a-WSi) superconducting nanowire single-photon detector (SNSPD). We performed laser induced fluorescence spectroscopy of surface adsorbates with sub-monolayer sensitivity and sub-nanosecond temporal resolution. We discuss possible future improvements of the SNSPD-based infrared emission spectrometer and its potential applications in molecular science.
Excited State Dynamics of 6-Thioguanine
Here we present the excited state dynamics of jet-cooled 6-thioguanine (6-TG), using resonance-enhanced multiphoton ionization (REMPI), IR–UV double resonance spectroscopy, and pump–probe spectroscopy in the nanosecond and picosecond time domains. We report data on two thiol tautomers, which appear to have different excited state dynamics. These decay to a dark state, possibly a triplet state, with rates depending on tautomer form and on excitation wavelength, with the fastest rate on the order of 1010 s–1. We also compare 6-TG with 9-enolguanine, for which we observed decay to a dark state with a 2 orders of magnitude smaller rate. At increased excitation energy (∼+500 cm–1) an additional pathway appears for the predominant thiol tautomer. Moreover, the excited state dynamics for 6-TG thiols is different from that recently predicted for thiones.
Nanoscale insights into doping behavior, particle size and surface effects in trivalent metal doped SnO2
Despite considerable research, the location of an aliovalent dopant into SnO2 nanoparticles is far to be clarified. The aim of the present study on trivalent lanthanide doped SnO2 is to differentiate between substitutional versus interstitial and surface versus bulk doping, delineate the bulk and surface defects induced by doping and establish an intrinsic dopant distribution. We evidence for the first time a complex distribution of intrinsic nature composed of substitutional isolated, substitutional associates with defects as well as surface centers. Such multi-modal distribution is revealed for Eu and Sm, while Pr, Tb and Dy appear to be distributed mostly on the SnO2 surface. Like the previously reported case of Eu, Sm displays a long-lived luminescence decaying in the hundreds of ms scale which is likely related to a selective interaction between the traps and the substitutional isolated center. Analyzing the time-gated luminescence, we conclude that the local lattice environment of the lattice Sn is not affected by the particle size, being remarkably similar in the ~2 and 20 nm particles. The photocatalytic measurements employed as a probe tool confirm the conclusions from the luminescence measurements concerning the nature of defects and the temperature induced migration of lanthanide dopants.
Non-Poissonian photon statistics from macroscopic photon cutting materials
In optical materials energy is usually extracted only from the lowest excited state, resulting in fundamental energy-efficiency limits such as the Shockley–Queisser limit for single-junction solar cells. Photon-cutting materials provide a way around such limits by absorbing high-energy photons and ‘cutting’ them into multiple low-energy excitations that can subsequently be extracted. The occurrence of photon cutting or quantum cutting has been demonstrated in a variety of materials, including semiconductor quantum dots, lanthanides and organic dyes. Here we show that photon cutting results in bunched photon emission on the timescale of the excited-state lifetime, even when observing a macroscopic number of optical centres. Our theoretical derivation matches well with experimental data on NaLaF4:Pr3+, a material that can cut deep-ultraviolet photons into two visible photons. This signature of photon cutting can be used to identify and characterize new photon-cutting materials unambiguously.
A cylindrical quadrupole ion trap in combination with an electrospray ion source for gas-phase luminescence and absorption spectroscopy
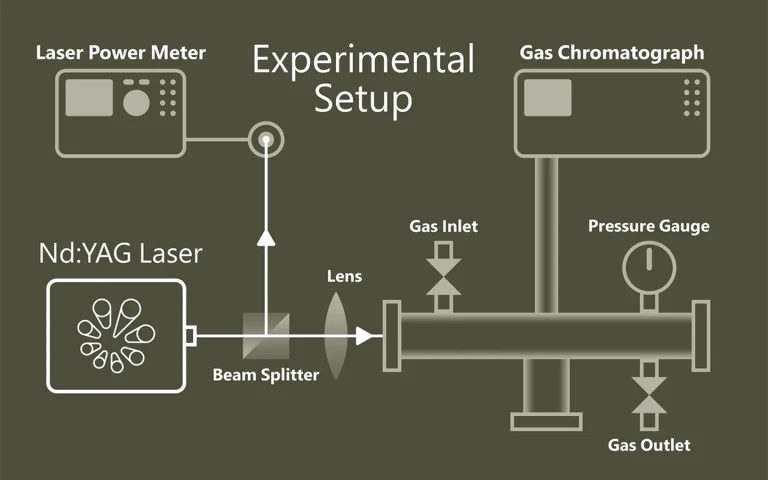
A relatively simple setup for collection and detection of light emitted from isolated photo-excited molecular ions has been constructed. It benefits from a high collection efficiency of photons, which is accomplished by using a cylindrical ion trap where one end-cap electrode is a mesh grid combined with an aspheric condenser lens. The geometry permits nearly 10% of the emitted light to be collected and, after transmission losses, approximately 5% to be delivered to the entrance of a grating spectrometer equipped with a detector array. The high collection efficiency enables the use of pulsed tunable lasers with low repetition rates (e.g., 20 Hz) instead of continuous wave (cw) lasers or very high repetition rate (e.g., MHz) lasers that are typically used as light sources for gas-phase fluorescence experiments on molecular ions. A hole has been drilled in the cylinder electrode so that a light pulse can interact with the ion cloud in the center of the trap. Simulations indicate that these modifications to the trap do not significantly affect the storage capability and the overall shape of the ion cloud. The overlap between the ion cloud and the laser light is basically 100%, and experimentally >50% of negatively charged chromophore ions are routinely photodepleted. The performance of the setup is illustrated based on fluorescence spectra of several laser dyes, and the quality of these spectra is comparable to those reported by other groups. Finally, by replacing the optical system with a channeltron detector, we demonstrate that the setup can also be used for gas-phase action spectroscopy where either depletion or fragmentation is monitored to provide an indirect measurement on the absorption spectrum of the ion.
Aerobic photoreactivity of synthetic eumelanins and pheomelanins: generation of singlet oxygen and superoxide anion
In this work, we examined photoreactivity of synthetic eumelanins, formed by autooxidation of DOPA, or enzymatic oxidation of 5,6-dihydroxyindole-2-carboxylic acid and synthetic pheomelanins obtained by enzymatic oxidation of 5-S-cysteinyldopa or 1:1 mixture of DOPA and cysteine. Electron paramagnetic resonance oximetry and spin trapping were used to measure oxygen consumption and formation of superoxide anion induced by irradiation of melanin with blue light, and time-resolved near-infrared luminescence was employed to determine the photoformation of singlet oxygen between 300 and 600 nm. Both superoxide anion and singlet oxygen were photogenerated by the synthetic melanins albeit with different efficiency. At 450-nm, quantum yield of singlet oxygen was very low (~10−4) but it strongly increased in the UV region. The melanins quenched singlet oxygen efficiently, indicating that photogeneration and quenching of singlet oxygen may play an important role in aerobic photochemistry of melanin pigments and could contribute to their photodegradation and photoaging.