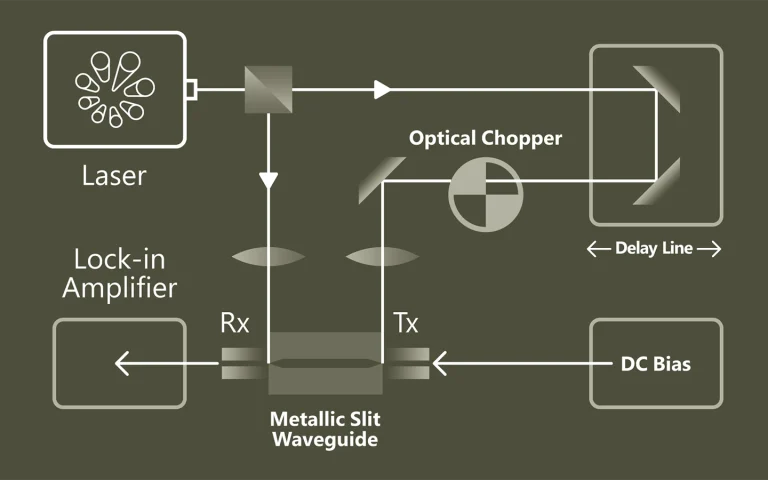
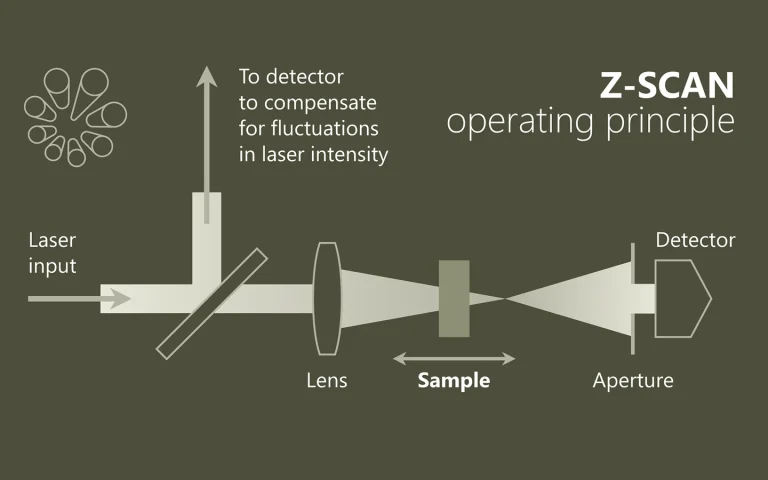
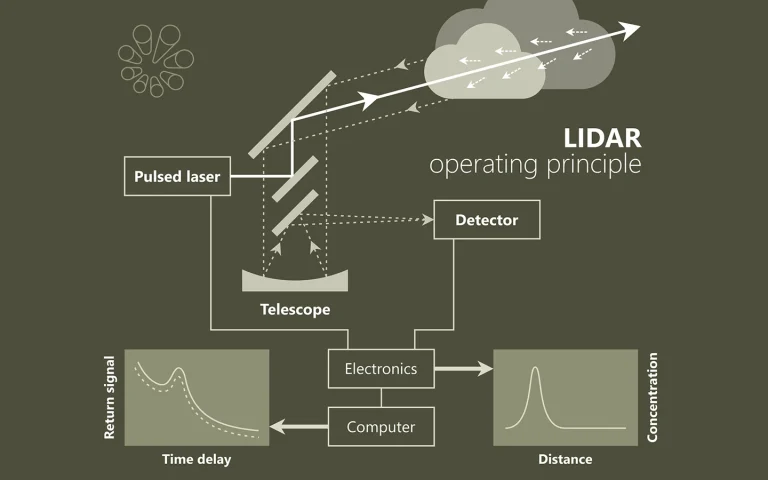
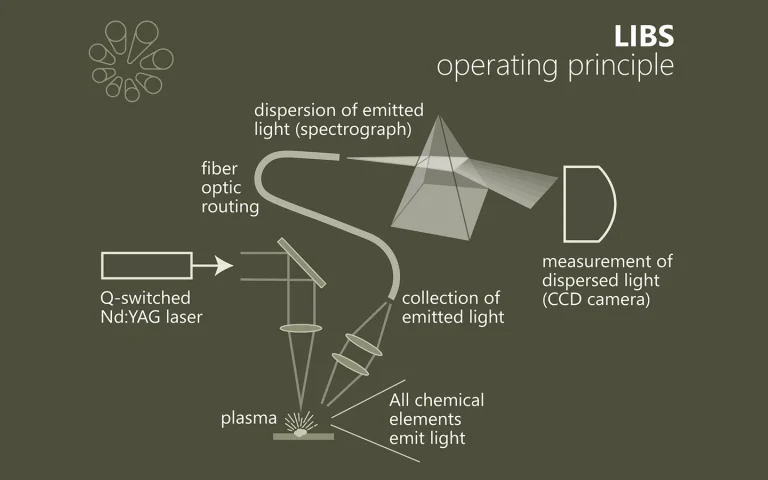
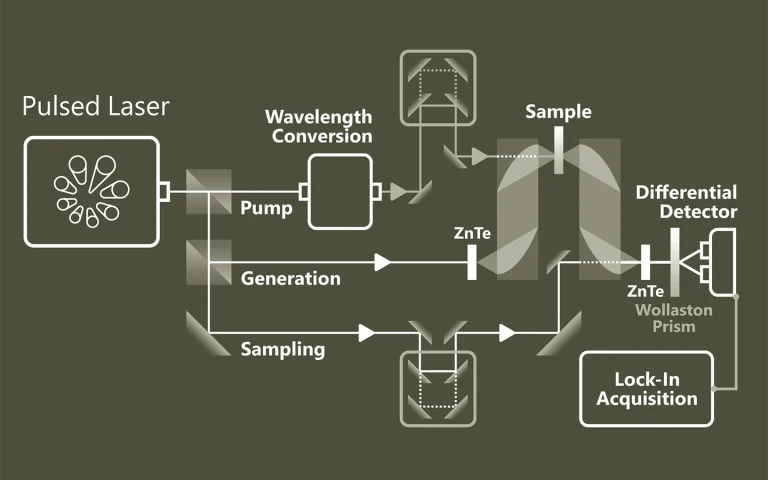
Laser spectroscopy
For a long time laser spectroscopy has been source of inspiration for EKSPLA scientific laser engineers. Explore some typical applications where our picosecond and nanosecond lasers has been employed.
Photolysis
Photodissociation, photolysis, or photodecomposition is a chemical reaction in which a chemical compound is broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photolysis plays an important role in photosynthesis, during which it produces energy by splitting water molecules into gaseous oxygen and hydrogen ions. This part of photosynthesis occurs in the granum of a chloroplast where light is absorbed by chlorophyll. This reacts with water and splits the oxygen and hydrogen molecules apart.
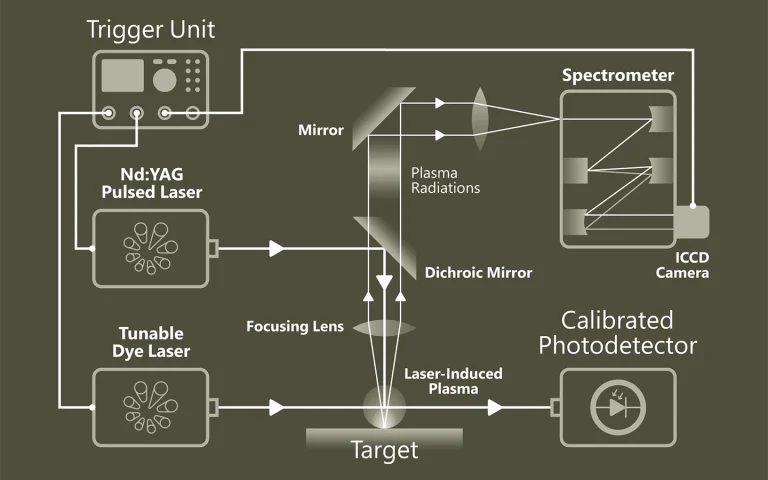
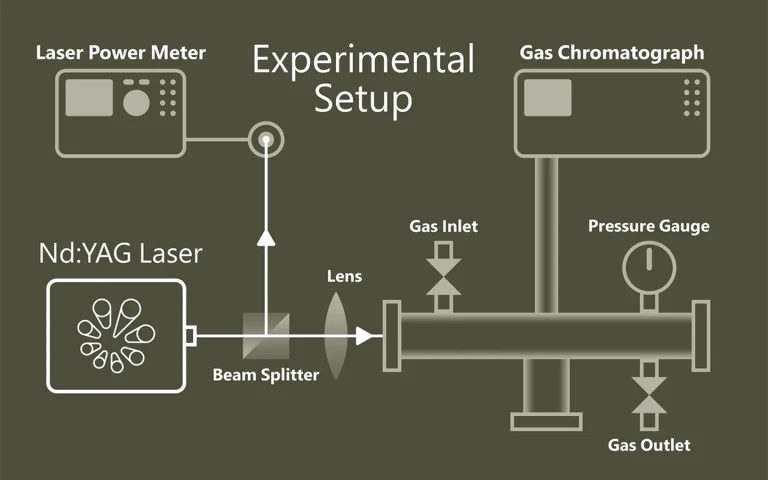
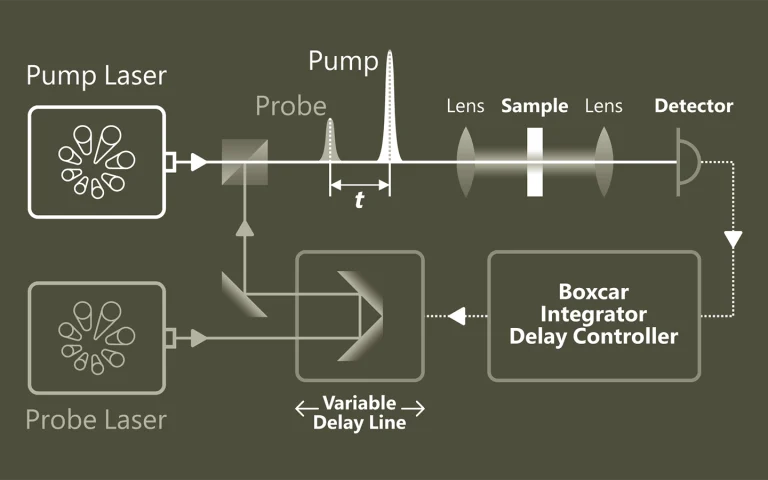
Laser Flash Photolysis is a technique for studying transient chemical and biological species generated by the short intense light pulse from a nanosecond/picosecond/femtosecond pulsed laser source (pump pulse). This intense light pulse creates short lived photo-excited intermediates such as excited states, radicals and ions. All these intermediates are generated in concentrations large enough for chemical and physical interaction to occur and for direct observation of the associated temporally changing absorption characteristics. Typically the absorption of light by the sample is recorded within short time intervals (by a so-called test or probe pulses) to monitor relaxation or reaction processes initiated by the pump pulse. Usage of Optical Parametric Oscillators (OPO) opens new possibilities in spectroscopic experiments.
Electronic spectroscopy and nanocalorimetry of hydrated magnesium ions [Mg(H2O)n]+, n = 20 – 70: spontaneous formation of a hydrated electron?
Hydrated singly charged magnesium ions [Mg(H2O)n]+ are thought to consist of an Mg2+ ion and a hydrated electron for n > 15. This idea is based on mass spectra, which exhibit a transition from [MgOH(H2O)n−1]+ to [Mg(H2O)n]+ around n = 15 – 22, black-body infrared radiative dissociation, and quantum chemical calculations. Here, we present photodissociation spectra of size-selected [Mg(H2O)n]+ in the range of n = 20 – 70 measured for photon energies of 1.0 – 5.0 eV. The spectra exhibit a broad absorption from 1.4 to 3.2 eV, with two local maxima around 1.7 – 1.8 eV and 2.1 – 2.5 eV, depending on cluster size. The spectra shift slowly from n = 20 to n = 50, but no significant change is observed for n = 50 – 70. Quantum chemical modeling of the spectra yields several candidates for the observed absorptions, including five- and six-fold coordinated Mg2+ with a hydrated electron in its immediate vicinity, as well as a solvent-separated Mg2+/e− pair. The photochemical behavior resembles that of the hydrated electron, with barrierless interconversion into the ground state following the excitation.
Photochemistry and spectroscopy of small hydrated magnesium clusters Mg+(H2O)n, n = 1 – 5
Hydrated singly charged magnesium ions Mg+(H2O)n, n ≤ 5, in the gas phase are ideal model systems to study photochemical hydrogen evolution since atomic hydrogen is formed over a wide range of wavelengths, with a strong cluster size dependence. Mass selected clusters are stored in the cell of an Fourier transform ion cyclotron resonance mass spectrometer at a temperature of 130 K for several seconds, which allows thermal equilibration via blackbody radiation. Tunable laser light is used for photodissociation. Strong transitions to D1 – 3 states (correlating with the 3s-3px,y,z transitions of Mg+) are observed for all cluster sizes, as well as a second absorption band at 4 – 5 eV for n = 3-5. Due to the lifted degeneracy of the 3px,y,z energy levels of Mg+, the absorptions are broad and red shifted with increasing coordination number of the Mg+ center, from 4.5 eV for n = 1 to 1.8 eV for n = 5. In all cases, H atom formation is the dominant photochemical reaction channel. Quantum chemical calculations using the full range of methods for excited state calculations reproduce the experimental spectra and explain all observed features. In particular, they show that H atom formation occurs in excited states, where the potential energy surface becomes repulsive along the O⋯H coordinate at relatively small distances. The loss of H2O, although thermochemically favorable, is a minor channel because, at least for the clusters n = 1-3, the conical intersection through which the system could relax to the electronic ground state is too high in energy. In some absorption bands, sequential absorption of multiple photons is required for photodissociation. For n = 1, these multiphoton spectra can be modeled on the basis of quantum chemical calculations.
Photodissociation of Sodium Iodide Clusters Doped with Small Hydrocarbons
Marine aerosols consist of a variety of compounds and play an important role in many atmospheric processes. In the present study, sodium iodide clusters with their simple isotope pattern serve as model systems for laboratory studies to investigate the role of iodide in the photochemical processing of sea-salt aerosols. Salt clusters doped with camphor, formate and pyruvate are studied in a Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR MS) coupled to a tunable laser system in both UV and IR range. The analysis is supported by ab initio calculations of absorption spectra and energetics of dissociative channels. We provide quantitative analysis of IRMPD measurements by reconstructing one-photon spectra and comparing them with the calculated ones. While neutral camphor is adsorbed on the cluster surface, the formate and pyruvate ions replace an iodide ion. The photodissociation spectra revealed several wavelength-specific fragmentation pathways, including the carbon dioxide radical anion formed by photolysis of pyruvate. Camphor and pyruvate doped clusters absorb in the spectral region above 290 nm, which is relevant for tropospheric photochemistry, leading to internal conversion followed by intramolecular vibrational redistribution, which leads to decomposition of the cluster. Potential photodissociation products of pyruvate in the actinic region may be formed with a cross section of <2×10−20 cm2, determined by the experimental noise level.
Infrared spectroscopy of O˙⁻OH⁻ in water clusters: evidence for fast interconversion between O˙⁻ and OH˙ OH⁻
We present infrared multiple photon dissociation (IRMPD) spectra of (H2O)nO˙− and (H2O)nOH− cluster ensembles for ñ ≈ 8 and 47 in the range of 2400 – 4000 cm−1. Both hydrated ions exhibit the same spectral features, in good agreement with theoretical calculations. Decomposition of the calculated spectra shows that bands originating from H2O⋯O˙− and H2O⋯OH− interactions span almost the whole spectral region of interest. Experimentally, evaporation of OH˙ is observed to a small extent, which requires interconversion of (H2O)nO˙− into (H2O)n–1OH˙OH−, with subsequent H2O evaporation preferred over OH˙ evaporation. The modeling shows that (H2O)nO˙− and (H2O)n–1OH˙OH− cannot be distinguished by IRMPD spectroscopy.
Photodissociation spectroscopy of protonated leucine enkephalin
Protonated leucine enkephalin (YGGFL) was studied by ultraviolet photodissociation (UVPD) from 225 to 300 nm utilizing an optical parametric oscillator tunable wavelength laser system (OPO). Fragments were identified by absolute mass measurement in a 9.4 T Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR MS). Bond cleavage was preferred in the vicinity of the two aromatic residues, resulting in high ion abundances for a4, a1, b3, y2 and y1 fragments. a, b and y ions dominated the mass spectrum, and full sequence coverage was achieved for those types. Photodissociation was most effective at the short wavelength end of the studied range, which is assigned to the onset of the La π–π* transition of the tyrosine chromophore, but worked well also at the Lb π–π* chromophore absorption maxima in the 35 000 – 39 000 cm−1 region. Several side-chain and internal fragments were observed. H atom loss is observed only above 41 000 cm−1, consistent with the requirement of a curve crossing to a repulsive 1πσ* state. It is suggested that the photochemically generated mobile H atom plays a role in further backbone cleavages, similar to the mechanism for electron capture dissociation. The b4 fragment is most intense at the Lb chromophore absorptions, undergoing additional fragmentation at higher photon energies. The high resolution of the FT-ICR MS revealed that out of all x and z-type fragments only x3 and x4 were formed, with low intensity. Other previously reported x- and z-fragments were re-assigned to internal fragments, based on exact mass measurement.