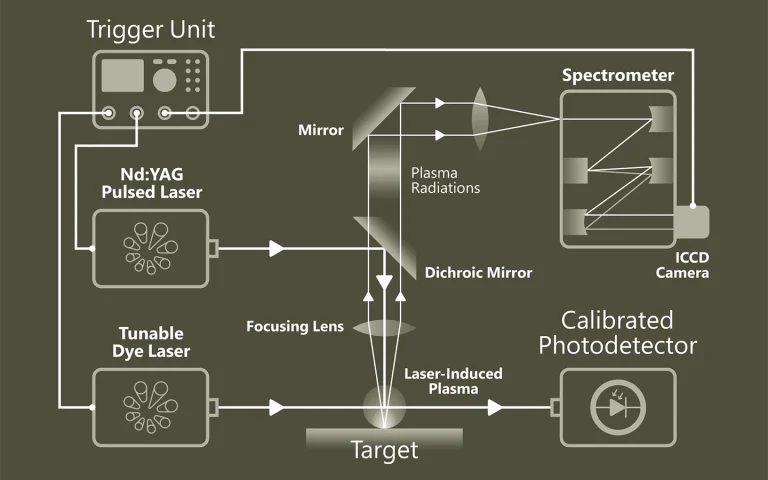
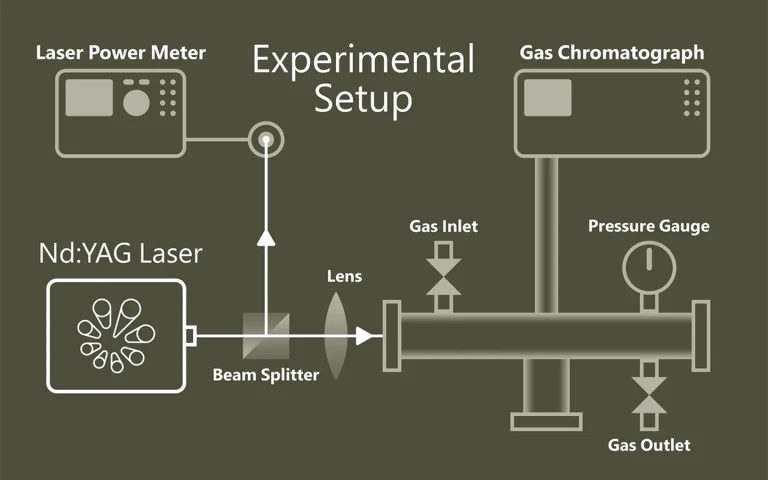
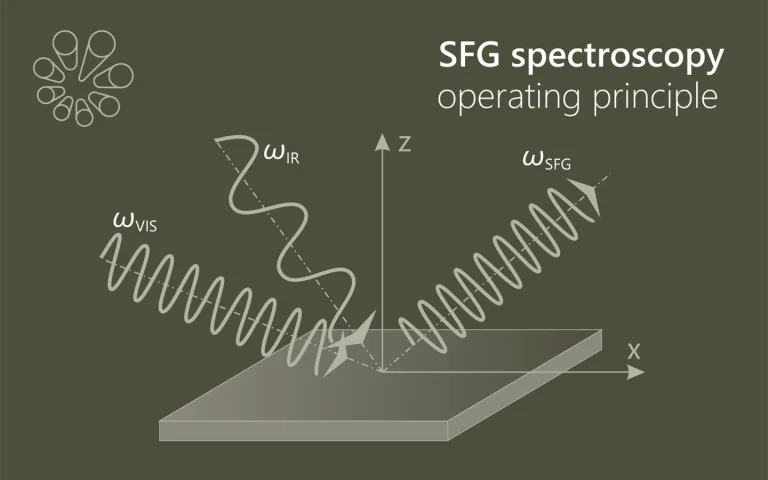
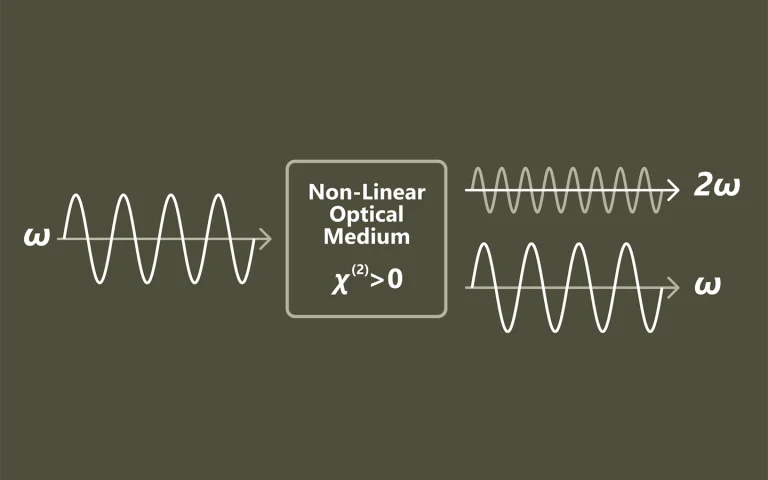
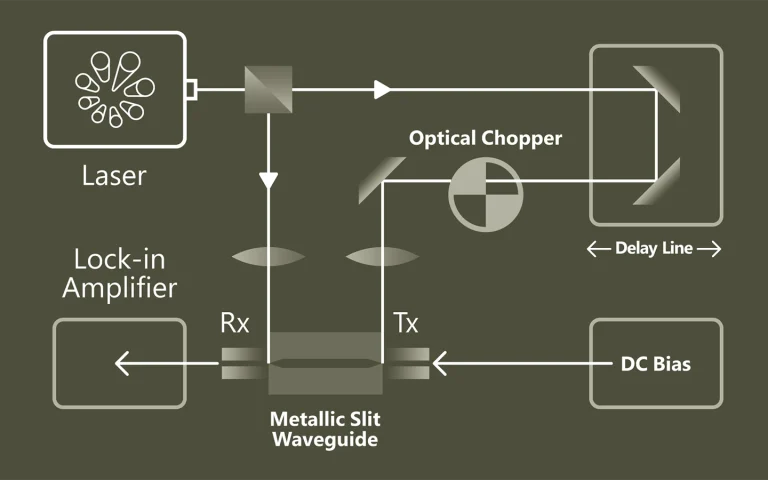
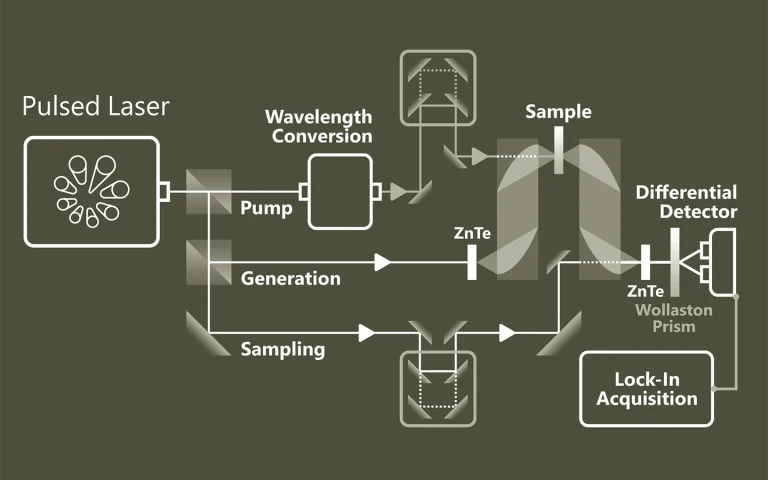
Laser spectroscopy
For a long time laser spectroscopy has been source of inspiration for EKSPLA scientific laser engineers. Explore some typical applications where our picosecond and nanosecond lasers has been employed.
Gas-phase ion luminescence spectroscopy
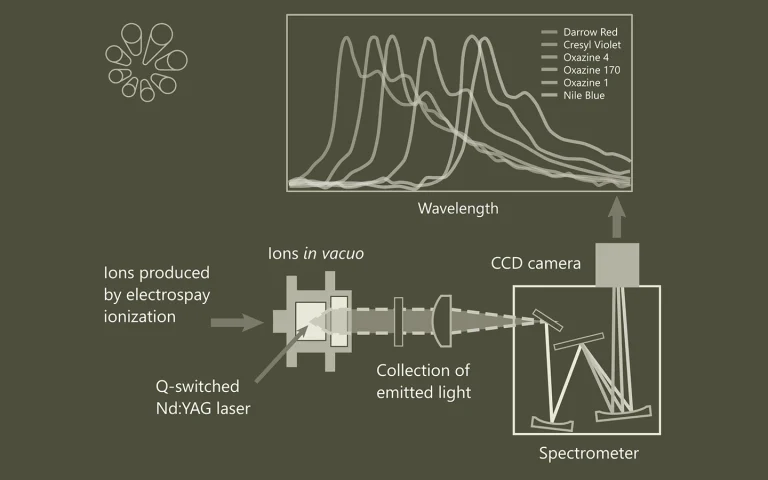
Gas-phase ion luminescence spectroscopy is used to determine intrinsic electronic transition energies in the absence of a disturbing environment. Large ions produced by electrospray ionization are stored and mass-selected in a cylindrical Paul trap. Here they are irradiated by light from a 20-Hz pulsed tunable wavelelength laser from EKSPLA followed by detection of the emitted photons. The 20-Hz repetition rate of the tunable laser allows for mass selection in between every irradiation event, which implies that there is no fluorescence contribution from ion impurities. The tunability of the laser makes it possible to photo-excite a variety of dyes that absorb at different wavelengths. The figure shows spectra of oxazine dye cations that display emissions in a range from 500 nm to 750 nm. The technique can also be used to study three-dimensional structures of peptides and nucleic acids in the gas phase based on Förster Resonance Energy Transfer (FRET). The biomolecules are labeled with donor-acceptor dye pairs such as rhodamines 575 and 640. Structure information is provided as the efficiency of energy transfer depends on the distance between the donor and acceptor to the inverse power of six.
Luminescence Spectroscopy of Rhodamine Homodimer Dications in Vacuo Reveals Strong Dye-Dye Interactions
Being alone or together makes a difference for the photophysics of dyes but for ionic dyes it is difficult to quantify the interactions due to solvent screening and nearby counter ions. Gas-phase luminescence experiments are desirable and now possible based on recent developments in mass spectrometry. Here we present results on tailor-made rhodamine homodimers where two dye cations are separated by methylene linkers, (CH2)n. In solution the fluorescence is almost identical to that from the monomer whereas the emission from bare cation dimers redshifts with decreasing n. In the absence of screening, the electric field from the charge on one dye is strong enough to polarize the other dye, both in the ground state and in the excited state. An electrostatic model based on symmetric dye responses (equal induced-dipole moments in ground state) captures the underlying physics and demonstrates interaction even at large distances. Our results have possible implications for gas-phase Förster Resonance Energy Transfer.
Gas-phase Ion Spectroscopy of Flexible and Nonflexible Nitrophenolates: Effect of Locking the Two Phenyl Units in 4’-nitro-[1,1’-biphenyl]-4-olate by a Bridging Atom
Nitrophenolates (NPs) are molecular anions that can undergo charge-transfer (CT) transitions determined by the degree of electron delocalization between the phenolate oxygen (donor group) and the nitro group (acceptor). Here we have studied four different NPs: 4’-nitro-[1,1’-biphenyl]-4-olate (1), 7-nitro-9H -carbazol-2-olate (NH linker, 2), 7-nitrodibenzo[b,d]furan-3-olate (oxygen linker, 3), and 7-nitrodibenzo[b,d]thiophen-3-olate (sulphur linker, 4), and recorded their electronic absorption spectra when isolated in vacuo to determine the effect of locking the biphenyl spacer group between
the donor and acceptor on transition energies. Absorption was identified from ion dissociation (action spectroscopy) using a homebuilt setup (sector mass spectrometer combined with pulsed laser). We find that the absorption is broad in the visible region for all four NPs with significant vibronic features. The lowest energy peak is at 601 ± 4 nm, 606 ± 4 nm, 615 ± 4 nm, and 620 ± 4 nm, for 3, 4, 2, and 1, respectively. NP 1 is flexible, and its lowest
energy structure is nonplanar while the other three NPs are planar according to density functional theory calculations. Hence in the case of 1 the electronic transition has a higher degree of CT than for the other three, accounting for its absorption furthest to the red. Our work demonstrates that oxygen and sulphur are best at conveying the electronic coupling between the donor and acceptor sites as 3 and 4 absorb furthest to the blue (i.e., the degree of CT is lowest for these two NPs). Based on the average spacing between the peaks in the vibrational progressions, coupling occurs to skeleton vibrational modes with frequencies of 649 ± 69 cm−1 (3), 655 ± 49 cm−1 (4), and 697 ± 52 cm−1 (2).
Luminescence spectroscopy of chalcogen substituted rhodamine cations in vacuo
Intrinsic optical properties of several rhodamine cations were probed by measuring their dispersed fluorescence spectra in vacuo. Three different rhodamine structures were investigated, each with four different chalcogen heteroatoms. Fluorescence band maxima were blue-shifted by between 0.15 and 0.20 eV (1200-1600 cm4) relative to previous solution-phase measurements. Trends in emission wavelengths and fluorescence quantum yields previously measured in solution are generally reproduced in the gas phase, confirming the intrinsic nature of these effects. One important exception is gas-phase brightness of the Texas Red analogues, which is significantly higher than the other rhodamine structures studied, despite having similar fluorescence quantum yields in solution. These results expand the library of fluorophores for which gas-phase photophysical data is available, and will aid in the design of experiments utilizing gas-phase structural biology methods such as Forster resonance energy transfer.
Sibling rivalry: intrinsic luminescence from two xanthene dye monoanions, resorufin and fluorescein, provides evidence for excited-state proton transfer in the latter
While the emission spectrum of fluorescein monoanions isolated in vacuo displays a broad and featureless band, that of resorufin, also belonging to the xanthene family, has a sharp band maximum, clear vibronic structure, and experiences a small Stokes shift. Excited-state proton transfer in fluorescein can account for the differences.