Laser spectroscopy & imaging
Decades of experience and close relationships with researchers enabled to create laser systems designed for specific laser spectroscopy and imaging applications.
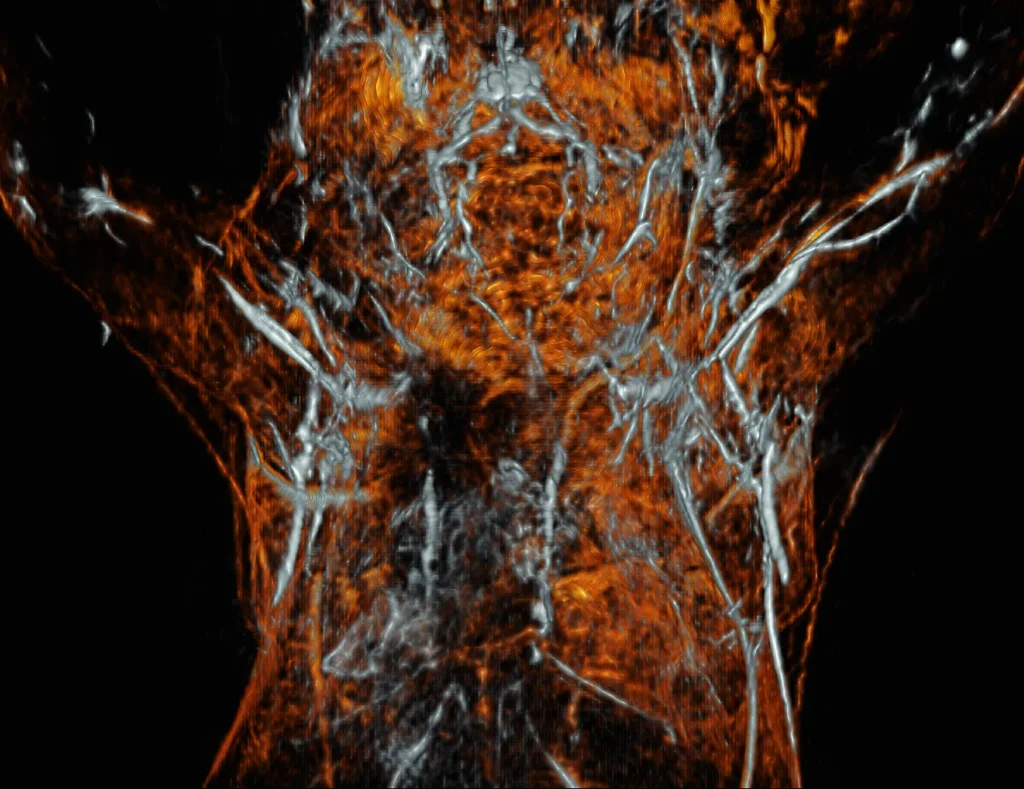
Laser sources for photoacoustic imaging
Photoacoustic imaging is one of the fastest-growing research areas
of non-invasive, high-resolution and high-contrast visualization of both superficial and deep tissues.
This method has a number of advantages over widely used conventional research and diagnostic methods as unlike X-ray, it does not use hazardous irradiation and has a significantly higher imaging resolution compared to conventional ultrasound. Photoacoustic imaging is proven to be very effective in diagnosing breast tumors, skin cancer, thyroid nodules, osteoarthritis and rheumatoid arthritis, early diagnosis of blood vessel disorders and many more. Photoacoustic imaging can also be used for visualization of non-living objects, such as nondestructive inspection of the internal structure and property changes of composite materials and food inspection.
Photoacoustic imaging employs the physical property of molecules to briefly heat up and cool down while absorbing a short pulse of light
(couple of nanoseconds) of a certain wavelength. While heating up, molecules expand and while cooling down, they contract. This creates an ultrasound wave which can be captured by ultrasound transducers enabling the ability to locate the origin of sound. The penetration of light into tissue depends on the tissue properties and the pulse energy of the light. Moreover, different chromophores in the tissue can absorb light of different wavelengths, thus giving functional visual information.
Comparison table of photoacoustic imaging sources
| Model | Available output wavelengths | Pulse duration 1) | Max repetition rate | Max pulse energy |
|---|---|---|---|---|
| Diode pumped laser source | ||||
| PhotoSonus X | 660 – 1300 nm (signal) 1065 – 2600 nm (idler) | 2 – 5 ns | 100 Hz | 90 mJ |
| Mobile flashlamp pumped laser source | ||||
| PhotoSonus M | 660 – 1320 nm (signal) 330 – 659 nm (SH) 1065 – 2300 nm (idler) | 3 – 5 ns | 20 Hz | 180 mJ |
| PhotoSonus M+ | 660 – 1064 nm (signal) 2) 330 – 530 nm (SH) 3) 1065 – 2300 nm (idler) | 3 – 5 ns | 10 Hz | 250 mJ |
| Table-top flashlamp pumped laser source | ||||
| PhotoSonus T | 660 – 1320 nm (signal) 330 – 659 nm (SH) 1065 – 2300 nm (idler) | 3 – 5 ns | 20 Hz | 150 mJ |
| PhotoSonus T+ | 660 – 1064 nm (signal) 2) 330 – 530 nm (SH) 3) 1065 – 2300 nm (idler) | 3 – 5 ns | 10 Hz | 230 mJ |
| Model | Available output wavelengths | Pulse duration 1) | Max repetition rate | Max pulse energy |
|---|
- FWHM measured with photodiode featuring 1 ns rise time and 300 MHz bandwidth oscilloscope.
- Optional signal extended range: 660 – 1320 nm.
- When extended signal range is selected, wavelength range is 330 – 659 nm.
Products range
Sum frequency generation spectroscopy systems
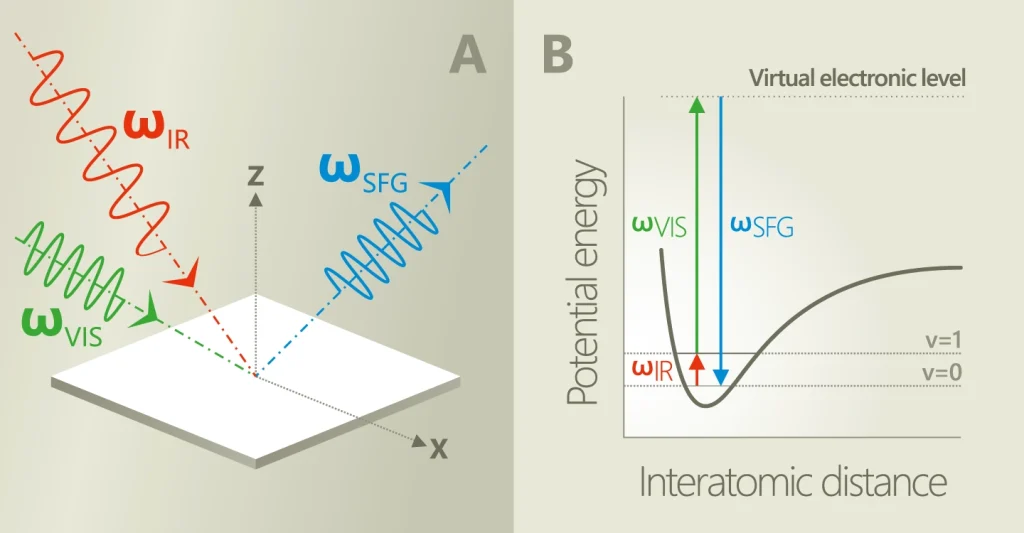
Vibrational Spectroscopy (SFG-VS) is powerful and versatile method for in-situ investigation of surfaces and interfaces. In SFG-VS experiment a pulsed tunable infrared IR (ωIR) laser beam is mixed with a visible VIS (ωVIS) beam to produce an output at the sum frequency (ωSFG = ωIR + ωVIS). SFG is second order nonlinear process, which is allowed only in media without inversion symmetry.
At surfaces or interfaces inversion symmetry is necessarily broken, that makes SFG highly surface specific. As the IR wavelength is scanned, active vibrational modes of molecules at the interface give a resonant contribution to SF signal. The resonant enhancement provides spectral information on surface characteristic vibrational transitions.
Vibrational sum frequency generation (SFG) spectroscopy holds several important advantages over traditional spectroscopy methods for the molecular level analysis of interfaces, including surface sensitivity, vibrational specificity, and the possibility to extract detailed information on the ordering and orientation of molecular groups at the interface by analysis of polarization-dependent SFG spectra.
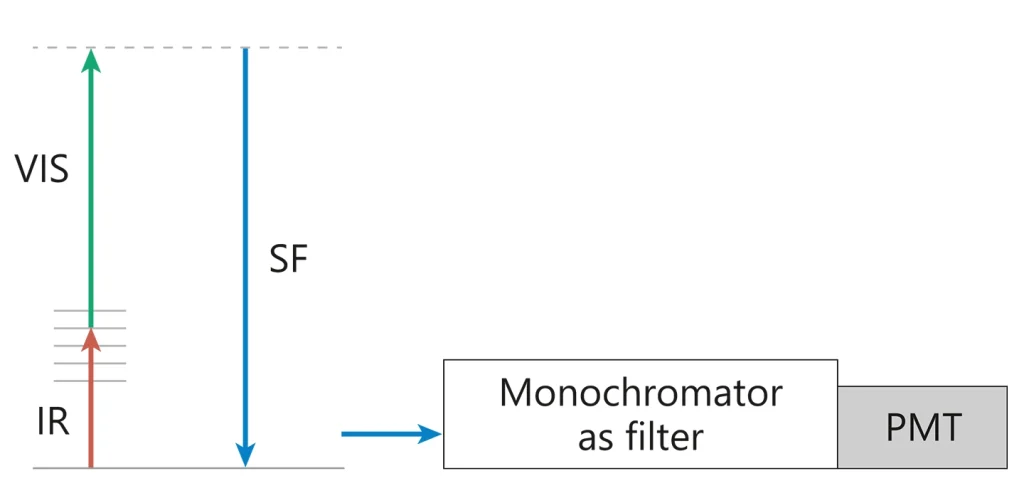
Narrowband picosecond scanning SFG spectrometer
In order to get SFG spectrum during measurement wavelength of narrowband mid-IR pulse is changed point-by-point throughout the range of interest. Narrowband SFG signal is recorded by the time-gated photomultiplier. Energy of each mid-IR, VIS and SFG pulse is measured. After the measurement, the SFG spectrum can be normalised according to IR and VIS energy. Spectral resolution is determined by the bandwidth of the mid-IR light source. The narrower mid-IR pulse bandwidth, the better the SFG spectral resolution. Separate vibrational modes are excited during the measurement.
Broadband femtosecond SFG spectrometer
A broadband mid‑IR pulse is mixed with a narrowband VIS pulse, producing a broadband SFG spectrum that is recorded using a monochromator and a sensitive CCD camera. The full spectrum is acquired simultaneously by integrating the signal over time. The spectral resolution is determined by the bandwidth of the VIS pulse and by the monochromator–camera combination. The narrower the bandwidth of VIS pulse, the better the SFG spectral resolution.
Comparison of different SFG spectrometres
| Narrowband Picosecond Scanning Spectrometer | Broadband Femtosecond High Resolution Spectrometer |
|---|---|
| Narrowband mid-IR excitation, only one band is excited. Coupled states can be separated. | Simultaneous exsitation and recording of broad vibration spectrum with high resolution. |
| High mid-IR pulse energy. Less influence of IR absorbtion in the air. | High mid-IR intensity at low pulse energy – suitable for biological or other water containing samples. |
| No reference spectrum needed, IR energy measured at each spectral point. | Optically coupled IR and VIS channels. Reduced complexity and increased stability of the system. |
Products range
Publications
A three-dimensional photoacoustic and ultrasound automated breast volume scanner (PAUS-ABVS) for breast cancer patients
Breast cancer screening with mammography is less effective in women with dense breast tissue, prompting the use of ultrasound (US) imaging. While two- (2D) and three-dimensional (3D) US improve cancer detection, their low specificity leads to frequent unnecessary biopsies. Operator dependence on 2D US has led to the development of 3D automated breast volume scanners (ABVS), but challenges remain in distinguishing benign from malignant lesions. We developed a 3D photoacoustic and ultrasound (PAUS)–ABVS system that integrates a large field-of-view, 768-element transducer to improve diagnostic accuracy. In a clinical study of 61 patients with 36 benign and 30 malignant lesions, multispectral photoacoustic imaging was used to measure blood volume and oxygen saturation within lesions. When combined with standard US BI-RADS (breast imaging reporting and data system) scores, the system achieved a sensitivity of 96.7% and specificity of 66.7%. This performance matched the best outcomes of 2D PAUS and outperformed conventional US. Our results suggest that the PAUS-ABVS can support more accurate breast cancer diagnosis while reducing unnecessary biopsies.
Au/Fe/Au trilayer nanodiscs as theranostic agents for magnet-guided photothermal, chemodynamic therapy and ferroptosis with photoacoustic imaging
Image-guided phototheranostics, including photothermal therapy and photoacoustic imaging using plasmonic nanoparticles, has attracted attention due to its remarkable photothermal conversion effects. In particular, considering the several benefits of biocompatibility, unique plasmon resonance, and tunable optical properties, gold nanoparticles of various shapes have been widely utilized as phototheranostic agents. However, applications in the near-infrared window have been limited due to the tendency of anisotropic gold nanoparticles to transform into spherical shapes under repetitive laser irradiation, which cause a shift in the localized surface plasmon resonance peak and reduces photothermal stability. To address these limitations, we introduce an Fe layer between Au nanodiscs to synthesize Au/Fe/Au trilayer uniform structured nanodiscs (AuFeAuNDs) using nanoimprint lithography. This approach aims to improve photostability and endow a magnetic targeting ability, enabling more accurate spatiotemporal regulation. The AuFeAuNDs primarily function as photothermal therapy agents and also serve as chemodynamic agents via the Fenton reaction specifically within the tumor microenvironment, which induces ferroptosis. Moreover, the AuFeAuNDs trigger immunogenic cell death following photothermal therapy. This study demonstrates applications of magnetic-targeted AuFeAuNDs for photoacoustic imaging-guided, spatiotemporal-controlled photothermal therapy, chemodynamic therapy, and immune modulation to bolster anti-tumor immune responses in cancer treatment.
Localized measurement of ultrasonic waves using a Fabry–Perot sensor illuminated by a Bessel beam
Fabry–Perot (FP) ultrasound sensors are a class of optical ultrasound sensors used in photoacoustic tomography (PAT) and other applications. Conventionally, an FP ultrasound sensor comprises an ultrasonically compressible planar microcavity, locally interrogated by a focused Gaussian beam. One way to increase the sensitivity could be to replace this beam with a Bessel beam. The rationale is twofold. First, as a Bessel beam’s wavefront better matches the modes of the planar microcavity, this could increase the -factor, leading to higher sensitivity. Second, as a Bessel beam provides a focused spatial structure—a central core surrounded by concentric rings—it might retain the ability to locally interrogate the sensor. To explore this idea, we developed an experimental system featuring a custom FP ultrasound sensor interrogated by a Bessel beam and evaluated its on-axis sensitivity, directivity, and image resolution when performing PAT. For comparison, we measured the same characteristics using a conventional focused Gaussian beam with a spot size similar to the core of the Bessel beam. As anticipated, the Bessel beam provided a higher ultrasonic on-axis sensitivity. However, the directivity and spatial resolution were degraded, suggesting that the Bessel beam yielded a larger acoustic element. We conclude that it is feasible to increase the sensitivity of an FP ultrasound sensor using a Bessel beam. Further work is required to establish whether differently designed Bessel beams could concurrently offer a smaller acoustic element.
Non-Invasive Photoacoustic Cerebrovascular Monitoring of Early-Stage Ischemic Strokes In Vivo
Abstract Early-stage stroke monitoring enables timely intervention that is crucial to minimizing neuronal damage and increasing the extent of recovery. By monitoring collateral circulation and neovascularization after ischemic stroke, the natural recovery process can be better understood, optimize further treatment strategies, and improve the prognosis. Photoacoustic computed tomography (PACT), a non-invasive imaging modality that captures multiparametric high-resolution images of vessel structures, is well suited for evaluating cerebrovascular structures and their function. Here 3D multiparametric transcranial PACT is implemented to monitor the early stage of a photothrombotic (PT)-stroke model in living rats. New vessels in the PT-induced region are successfully observed using PACT, and these observations are confirmed by histology. Then, using multiparametric PACT, it is found that the SO2 in the ischemic area decreases while the SO2 in newly formed vessels increases, and the SO2 in the PT region also recovers. These findings demonstrate PACT\’s remarkable ability to image and monitor cerebrovascular morphologic and physiological changes. They highlight the usefulness of whole-brain PACT as a potentially powerful tool for early diagnosis and therapeutic decision-making in treating ischemic stroke.
Three-Dimensional Whole-Body Small Animal Photoacoustic Tomography Using a Multi-View Fabry-Perot Scanner
Photoacoustic tomography (PAT) has the potential to become a widely used imaging tool in preclinical studies of small animals. This is because it can provide non-invasive, label free images of whole-body mouse anatomy, in a manner which is challenging for more established imaging modalities. However, existing PAT scanners are limited because they either do not implement a full 3-D tomographic reconstruction using all the recorded photoacoustic (PA) data and/or do not record the available 3-D PA time-series data around the mouse with sufficiently high spatial resolution ( ∼100μ m), which compromises image quality in terms of resolution, imaging depth and the introduction of artefacts. In this study, we address these limitations by demonstrating an all-optical, multi-view Fabry-Perot based scanner for whole body small animal imaging. The scanner densely samples the acoustic field with a large number of detection points (>100,000), evenly distributed around the mouse. The locations of the detection points were registered onto a common coordinate system, before a tomographic reconstruction using all the recorded PA time series was implemented. This enabled the acquisition of high resolution, whole-body PAT images of ex-vivo mice, with anatomical features visible across the entire cross section.
A fast all-optical 3D photoacoustic scanner for clinical vascular imaging
The clinical assessment of microvascular pathologies (in diabetes and in inflammatory skin diseases, for example) requires the visualization of superficial vascular anatomy. Photoacoustic tomography (PAT) scanners based on an all-optical Fabry–Perot ultrasound sensor can provide highly detailed 3D microvascular images, but minutes-long acquisition times have precluded their clinical use. Here we show that scan times can be reduced to a few seconds and even hundreds of milliseconds by parallelizing the optical architecture of the sensor readout, by using excitation lasers with high pulse-repetition frequencies and by exploiting compressed sensing. A PAT scanner with such fast acquisition minimizes motion-related artefacts and allows for the volumetric visualization of individual arterioles, venules, venous valves and millimetre-scale arteries and veins to depths approaching 15 mm, as well as for dynamic 3D images of time-varying tissue perfusion and other haemodynamic events. In exploratory case studies, we used the scanner to visualize and quantify microvascular changes associated with peripheral vascular disease, skin inflammation and rheumatoid arthritis. Fast all-optical PAT may prove useful in cardiovascular medicine, oncology, dermatology and rheumatology.
Analysis of characteristics and photoacoustic imaging performance of exogenous contrast agents
Photoacoustic imaging offers high spatial resolution, imaging depth, and molecular information, emerging as a promising biomedical imaging modality. In particular, when using exogenous contrast, the advantages of photoacoustic imaging can be more effectively utilized in preclinical and clinical studies. We provide a novel approach to screen and identify efficient photoacoustic performance as contrast agents of the metals. To accomplish this, we introduce a novel figure of merit that quantifies the potential performance of contrast agents. As a result of the quantification, we discover that Ti nanodiscs outperform Pt nanodiscs in terms of photoacoustic ability, which shows a similar level to Au nanodiscs. We compare these results by performing a photoacoustic phantom imaging experiment. The photoacoustic performance of the three materials is compared by comparing the signal intensity of the materials measured on the photoacoustic image for various wavelengths. The imaging results further support our findings, demonstrating the superior performance of Ti nanodiscs as contrast agents.
Exploring salinity induced adaptations in marine diatoms using advanced photonic techniques
Photonic-based methods are crucial in biology and medicine due to their non-invasive nature, allowing remote measurements without affecting biological specimens. The study of diatoms using advanced photonic methods remains a relatively underexplored area, presenting significant opportunities for pioneering discoveries. This research provides a comprehensive analysis of marine diatoms, specifically Nitzschia sp., across varying salinity levels, integrating fluorescence lifetime imaging microscopy (FLIM), combined photoacoustic and fluorescence tomographies (PAFT), and ultrastructural examinations using transmission electron microscopy. Key findings include a systematic shift in the mean fluorescence lifetime from 570 ps at 20‰ to 940 ps at 80‰, indicating functional adaptations in chlorophyll molecules within light-harvesting complexes. At 60‰ salinity, anomalies are observed in the development of silica valves and polysaccharide layers, suggesting abnormalities in valve morphogenesis. Lipid droplets within the cells display a minimum diameter at 40‰, indicating metabolic adjustments to osmotic stress. The intensity of both fluorescence and photoacoustic signals increases with increasing salinity levels. These insights enhance understanding of the ecological implications of salinity stress on diatom communities and pave the way for future research on leveraging the unique adaptive mechanisms of microalgae for environmental monitoring and sustainable biotechnological applications.
Hybrid Photoacoustic Ultrasound Imaging System for Cold-Induced Vasoconstriction and Vasodilation Monitoring
Lewis hunting reaction refers to the alternating cold-induced vasoconstriction and dilation in extremities, whose underlying mechanism is complex. While numerous studies reported this intriguing phenomenon by measuring cutaneous temperature fluctuation under cold exposure, few of them tracked peripheral vascular responses in real-time, lacking a non-invasive and quantitative imaging tool. To better monitor hunting reaction and diagnose relevant diseases, we developed a hybrid photoacoustic ultrasound (PAUS) tomography system to monitor finger vessels’ dynamic response to cold, together with simultaneous temperature measurement. We also came out a standard workflow for image analysis with self-defined indices. In the small cohort observational study, vascular changes in the first cycle of hunting reaction were successfully captured by the image series and quantified. Time difference between vasodilation and temperature recovery was noticed and reported for the first time, thanks to the unique capability of the PAUS imaging system in real-time and continuous vascular monitoring. The developed imaging system and indices enabled more objective and quantitative monitoring of peripheral vascular activities, indicating its great potential in numerous clinical applications.
Video-rate endocavity photoacoustic/harmonic ultrasound imaging with miniaturized light delivery
SignificanceEndocavity ultrasound (US) imaging is a frequently employed diagnostic technique in gynecology and urology for the assessment of male and female genital diseases that present challenges for conventional transabdominal imaging. The integration of photoacoustic (PA) imaging with clinical US imaging has displayed promising outcomes in clinical research. Nonetheless, its application has been constrained due to size limitations, restricting it to spatially confined locations such as vaginal or rectal canals.AimThis study presents the development of a video-rate (20 Hz) endocavity PA/harmonic US imaging (EPAUSI) system.ApproachThe approach incorporates a commercially available endocavity US probe with a miniaturized laser delivery unit, comprised of a single large-core fiber and a line beamshaping engineered diffuser. The system facilitates real-time image display and subsequent processing, including angular energy density correction and spectral unmixing, in offline mode.ResultsThe spatial resolutions of the concurrently acquired PA and harmonic US images were measured at 318 μm and 291 μm in the radial direction, respectively, and 1.22 deg and 1.50 deg in the angular direction, respectively. Furthermore, the system demonstrated its capability in multispectral PA imaging by successfully distinguishing two clinical dyes in a tissue-mimicking phantom. Its rapid temporal resolution enabled the capture of kinetic dye perfusion into an ex vivo porcine ovary through the depth of porcine uterine tissue. EPAUSI proved its clinical viability by detecting pulsating hemodynamics in the male rat’s prostate in vivo and accurately classifying human blood vessels into arteries and veins based on sO2 measurements.ConclusionsOur proposed EPAUSI system holds the potential to unveil previously overlooked indicators of vascular alterations in genital cancers or endometriosis, addressing pressing requirements in the fields of gynecology and urology.